What Is Clinical Data Management?
Definition of clinical data management
Clinical Data Management (CDM) refers to the structured process of collecting, validating, cleaning, storing, and managing data generated throughout clinical trials and healthcare operations. Its primary objective is to ensure that clinical data is accurate, complete, consistent, and compliant with regulatory standards, so it can be trusted for medical, operational, and strategic decisions.
Role of Clinical Data Management in Clinical Trials
In clinical trials, CDM serves as the backbone of the entire research lifecycle. It supports:
-
Reliable trial results by maintaining high data integrity
-
Regulatory submissions through validated and audit-ready datasets
-
Faster trial timelines by reducing rework and manual intervention
-
Risk mitigation by identifying data issues early
Ineffective data management can lead to delayed trials, regulatory findings, increased costs, or even trial failure, making CDM a critical concern for sponsors and executive stakeholders.
Role of Clinical Data Management in Healthcare Operations
Beyond clinical trials, CDM plays a vital role in day-to-day healthcare operations. It enables organizations to:
-
Consolidate and manage large volumes of patient and operational data
-
Support clinical decision-making and care optimization
-
Enable analytics, reporting, and performance monitoring
-
Maintain compliance with healthcare regulations and data governance policies
As healthcare systems become more digital and data-intensive, clinical data management increasingly determines how effectively organizations can scale operations, control costs, and leverage data as a strategic asset.
Types of Clinical Data
Clinical data management encompasses a vast, ever-expanding array of data sources, each with unique formats, requirements, and compliance considerations. Mastering these types is crucial for AI in clinical data management teams seeking to implement robust, transformative solutions.
Patient Records and EHR Data
Patient records and Electronic Health Records (EHRs) capture longitudinal data related to patient care, including demographics, diagnoses, treatment histories, medications, and clinical notes.
From a management standpoint, EHR data presents several challenges:
-
Data is often distributed across multiple systems and providers
-
Clinical notes are largely unstructured, limiting traditional analytics
-
Inconsistencies in data entry reduce data reliability
-
Privacy and security requirements increase governance complexity
Despite these challenges, EHR data is one of the most valuable assets for improving care quality, operational efficiency, and population health, if it can be effectively managed and analyzed.
Clinical Trial Data
Clinical trial data is generated throughout the lifecycle of a trial, from study design and patient enrollment to monitoring and final analysis. This data typically includes:
-
Case Report Forms (CRFs)
-
Patient-reported outcomes
-
Adverse event reports
-
Protocol deviations and monitoring data
Clinical trial data must meet the highest standards of accuracy, traceability, and regulatory compliance. Even minor errors or inconsistencies can delay approvals, trigger audits, or compromise trial credibility.
Imaging, Lab Results, and Real-World Data
In addition to structured clinical and trial data, healthcare organizations increasingly manage high-volume and high-complexity data sources, including:
-
Medical imaging (X-rays, CT scans, MRIs)
-
Laboratory test results and biomarker data
-
Real-world data (RWD) from wearables, registries, and patient-reported sources
These data types are often data-intensive, unstructured, and generated in near real time. When effectively integrated with traditional clinical datasets, they provide richer insights into treatment effectiveness, safety, and patient outcomes.
However, without advanced technologies such as AI, extracting value from these datasets is slow, costly, and difficult to scale.
⭐️ Learn more about Healthcare Software Development Guide
 What AI Technologies Are Used in Clinical Data Management?
What AI Technologies Are Used in Clinical Data Management?
To truly harness AI in clinical data management, organizations must understand the core technologies underpinning modern automation, insight, and efficiency. Each tech stack component solves a specific workflow challenge in the clinical data journey.
Machine Learning (ML)
Machine Learning enables systems to learn from historical clinical data and continuously improve data quality and operational efficiency over time.
In clinical data management, ML is commonly used to:
-
Detect data anomalies, inconsistencies, and outliers at scale
-
Predict missing or erroneous values based on historical patterns
-
Identify trends and risks in clinical trial data early
-
Support advanced analytics and forecasting
Natural Language Processing (NLP)
A significant portion of clinical data exists in unstructured text, such as physician notes, discharge summaries, and adverse event narratives. Natural Language Processing enables systems to interpret and structure this information.
Key applications of NLP include:
-
Extracting clinical entities from free-text records
-
Structuring patient notes and trial documentation
-
Automating coding and classification tasks
-
Improving data consistency across systems
Computer Vision
Computer vision allows AI systems to analyze and interpret visual data, making it particularly valuable for imaging-intensive clinical environments.
In clinical data management, computer vision supports:
-
Automated analysis of medical images (e.g., radiology, pathology)
-
Quality control and standardization of imaging data
-
Detection of anomalies or patterns not easily identified by human review
By reducing manual image review and improving consistency, computer vision enhances both operational efficiency and data reliability.
Intelligent Automation
Intelligent automation combines AI technologies with workflow automation to streamline end-to-end clinical data processes.
Typical use cases include:
-
Automated data ingestion and validation
-
Workflow orchestration across clinical systems
-
Continuous data monitoring and exception handling
-
Reduction of repetitive, rule-based manual tasks
⭐️ Learn how to create an AI agent to build autonomous AI that drives real business impact
Business Benefits of AI in Clinical Data Management
For clinical operations leaders, R&D sponsors, and regulatory professionals, the business case for AI in clinical data management is increasingly impossible to ignore. Quantifiable improvements in speed, accuracy, compliance, and cost efficiency are defining the new operational standard.
Improved Data Quality and Decision Confidence
AI significantly enhances data accuracy, consistency, and completeness by continuously detecting anomalies, inconsistencies, and missing values across large datasets.
For executives, this translates into:
-
Higher confidence in clinical and operational decisions
-
Reduced risk of errors impacting trial outcomes or patient care
-
More reliable analytics and reporting at the leadership level
Cost Optimization Through Automation
Manual data cleaning, validation, and reconciliation are among the most resource-intensive activities in clinical data management. AI-driven automation reduces these efforts dramatically.
Business impact includes:
-
Lower operational and labor costs
-
Reduced dependency on manual review processes
-
More efficient allocation of skilled clinical and data professionals
Faster Time-to-Market and Operational Speed
Speed is a competitive advantage in healthcare and life sciences. AI accelerates data processing, monitoring, and analysis, shortening clinical trial timelines and operational cycles.
For decision-makers, this means:
-
Faster clinical trial execution
-
Quicker regulatory submissions
-
Accelerated innovation and commercialization
Stronger Regulatory Compliance and Risk Reduction
Regulatory scrutiny around clinical data continues to intensify. AI supports continuous data monitoring, audit trails, and compliance checks throughout the data lifecycle.
Key benefits include:
-
Improved audit readiness and traceability
-
Early detection of compliance risks
-
Reduced likelihood of regulatory delays or findings
Scalability and Long-Term Competitive Advantage
As data volumes grow and clinical environments become more complex, traditional data management models struggle to scale. AI provides the flexibility and intelligence needed to manage increasing complexity without proportional cost increases.
Strategic advantages include:
-
Scalable operations across multiple trials or facilities
-
Better use of real-world and advanced data sources
-
A stronger data foundation for future digital transformation initiatives
Key Use Cases of AI in Clinical Data Management
AI is already transforming frontline CDM workflows across research sites, sponsors, and hospitals. These use cases exemplify the practical, measurable value created by AI in clinical data management in action.
Automated Data Cleaning and Validation
AI enables continuous, automated validation of clinical data across multiple sources and systems. Instead of relying on manual checks and retrospective reviews, AI models identify anomalies, inconsistencies, and missing values in near real time.
Typical applications include:
-
Automated detection of outliers and data discrepancies
-
Rule-based and learning-based validation checks
-
Reduction of manual data review cycles
For example, Pfizer has publicly discussed its use of advanced analytics and AI-driven data quality checks to support large, global clinical trials. By applying machine learning models to identify unusual patterns in patient and site data, Pfizer improves data integrity and reduces the time required for manual data cleaning and query resolution.
Intelligent Data Extraction and Structuring
A large portion of clinical data is unstructured, embedded in physician notes, reports, and trial documentation. AI, particularly NLP, can extract relevant clinical entities and convert them into structured, analyzable formats.
Common use cases include:
-
Extracting key variables from clinical narratives
-
Structuring patient-reported outcomes and adverse event descriptions
-
Standardizing data across heterogeneous sources
A common challenge across data-driven industries is turning complex, unstructured visual data into accurate, actionable insights at scale. Ekotek addressed this problem by building a Beauty AI application that uses AI-powered image analysis to automatically assess skin conditions and deliver personalized recommendations in real time, eliminating manual evaluation and subjective judgment. This solution demonstrates how AI can standardize analysis, improve decision accuracy, and scale personalized experiences efficiently.
⭐️ Explore Ekotek’s Beauty AI case study
Real-Time Data Monitoring and Risk Detection
AI-powered monitoring systems continuously analyze incoming clinical data to identify risks, trends, and deviations as they emerge.
Key applications include:
-
Early detection of protocol deviations
-
Identification of data quality or compliance risks
-
Real-time alerts for unusual patterns or adverse signals
For example, Novartis applies AI-driven monitoring and analytics to enhance oversight of clinical trials. By analyzing incoming trial data across sites, AI systems help detect unusual trends or deviations early, allowing teams to intervene before issues escalate into regulatory or safety risks.
Clinical Trial Acceleration
By integrating multiple AI capabilities, organizations can significantly accelerate clinical trial execution. AI supports faster patient data processing, improved monitoring, and more efficient trial oversight.
Business-relevant outcomes include:
-
Shortened trial timelines
-
Faster decision-making during trial execution
-
Improved coordination across trial sites and stakeholders
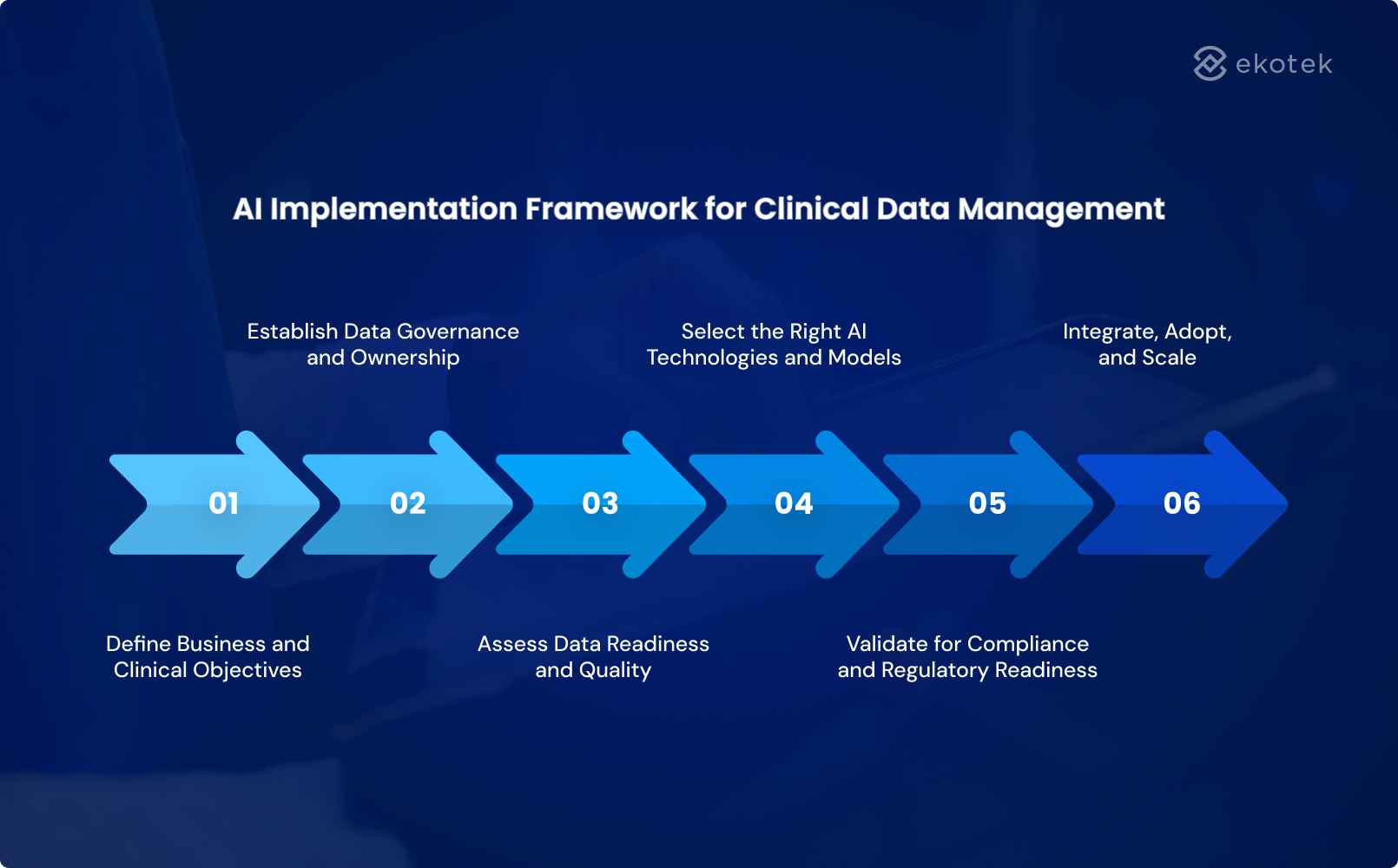
AI Implementation Framework for Clinical Data Management
Adopting AI in clinical data management requires far more than technology: it demands a phased, strategic approach that aligns systems, people, and compliance structures for maximum, sustainable value.
Define Business and Clinical Objectives
AI initiatives must start with clearly defined objectives that align clinical priorities with business outcomes. Without this alignment, AI risks becoming a technology experiment rather than a value driver.
Key questions for decision-makers include:
-
What business problems are we solving (cost, speed, risk, scalability)?
-
Which clinical processes will benefit most from AI?
-
How will success be measured (ROI, cycle time reduction, data quality metrics)?
Establish Data Governance and Ownership
Clinical data is highly regulated, sensitive, and often distributed across systems and teams. Strong governance is essential to ensure accountability, trust, and compliance.
This step includes:
-
Defining data ownership and stewardship roles
-
Establishing data access, privacy, and security policies
-
Aligning governance with regulatory requirements and internal controls
Assess Data Readiness and Quality
AI performance is only as strong as the data it relies on. Before implementation, organizations must assess whether their data is fit for AI-driven use cases.
Key considerations:
-
Data completeness, consistency, and accuracy
-
Level of structure vs. unstructured data
-
Data integration across systems and sources
Select the Right AI Technologies and Models
Not all AI technologies are suitable for every clinical data challenge. Leaders should focus on selecting technologies that directly support defined objectives.
This includes:
-
Choosing appropriate AI approaches (ML, NLP, automation, computer vision)
-
Balancing model complexity with transparency and explainability
-
Ensuring scalability across studies, sites, or healthcare operations
Validate for Compliance and Regulatory Readiness
In clinical environments, AI must meet strict regulatory and compliance standards. Validation is a critical step, not an afterthought.
Key activities include:
-
Ensuring traceability and auditability of AI-driven decisions
-
Validating models according to regulatory guidelines
-
Documenting processes for inspections and audits
Integrate, Adopt, and Scale
The final and often most challenging phase is operationalizing AI across the organization.
This involves:
-
Integrating AI solutions with existing clinical systems and workflows
-
Driving adoption through change management and training
-
Scaling successful use cases across departments, trials, or facilities
⭐️ Accelerate your AI roadmap with AI outsourcing
 Conclusion
Conclusion
AI in clinical data management has moved beyond experimentation to become a strategic capability for healthcare and life sciences organizations. By improving data quality, accelerating clinical operations, and strengthening regulatory readiness, AI enables leaders to make faster, more confident, and more informed decisions. Organizations that approach AI with a clear implementation framework and strong business alignment are better positioned to scale innovation and sustain long-term competitive advantage.
Turning this potential into real-world outcomes requires not only the right technology, but also the right partner. Ekotek is a leading software development company specializing in digital transformation, AI, and blockchain development. With a team of more than 200 professionals who possess deep expertise in AI, Ekotek has delivered impactful solutions across multiple industries, including healthcare, manufacturing, logistics, banking, and finance.
Ekotek provides a comprehensive range of AI services, spanning computer vision, AI integration, AI chatbots, generative AI, and agentic AI, built to address complex data and operational challenges. In addition to custom-built solutions, Ekotek also offers ready-made AI products that enable faster time to market while remaining flexible enough to be tailored to each organization’s specific clinical and business needs.
Looking to leverage AI in clinical data management?
Explore how AI-powered solutions can accelerate your digital transformation journey and deliver measurable results

 Conclusion
Conclusion
